Case Study
Rachel Gilroy, Product Development Intern Testimonial
"Coming to the end of your PhD marks a time when you need to make decisions on how you want to progress your future career. Making the move from academia to a career in industry is undeniably daunting, and with no industry connections, it can be difficult to take that first step. For me, I was keen to explore the veterinary pharmaceutical industry, and when I attended a talk given by Dechra at Durham University about career options of veterinarians in industry with focus on R&D, it provided me with an invaluable opportunity to approach Dechra regarding an internship in R&D. Having a long established history within the animal health market alongside a fundamental values system, Dechra represented a growing global market brand able to offer a unique internship experience.
Funded as part of the fully integrated BBSRC DTP PhD Internship programme, my three month placement was based primarily in the Product Development offices in Shrewsbury, UK.
During the initial phase of my internship, tasks were mainly focused on exploring published research to generate a comprehensive literature review which would determine subsequent clinical trial protocols. Moreover, I was involved in the creation of a technical training presentation which would be used for the roll out of a referencing software throughout the Group. Importantly it was during this early stage of my internship that great effort was made by all members of the Dechra team, both UK and international, to introduce themselves, their role and their wealth of previous experience. Not only did this make me feel particularly welcome but was valuable in illustrating how different industry roles are not defined by a single career path.
With a clinical team based largely in the US, Dechra allowed me the opportunity to visit teams in both Maine and Kansas. Working closely with the clinical team in this way gave me tangible experience with procedures and software that formed the basis of clinical operations. Having one-to-one discussions with colleagues working in areas including regulatory affairs, safety and marketing gave me an appreciation of the multi-faceted nature of drug discovery and development, and how each role interlinks with another.
Most notably, I was heavily involved in the formation of a final study report for the submission of pilot clinical trial data to the FDA. Not only did this allow me to assess critically and interpret clinical data, but also present this in a way that complies with current guidelines. Dechra gave me the independence of being able to approach tasks in a manner that suited my skill set, while offering continued mentorship and encouragement during processes with which I was particularly unfamiliar. Being able to participate in clinical operations review meetings highlighted fundamental drug development milestones and challenges, but more importantly, how these are handled.
The ability to overlap my internship with my PhD has allowed me to apply some of the project management and trial protocol techniques to my ongoing research. Furthermore, I was able to present my current PhD research to teams in both the Netherlands and Croatia, sharing knowledge and feedback that could develop my research to application in both academia and industry.
Before working with Dechra, I had little sense of product development processes in the veterinary pharmaceutical industry. Thanks to the dedicated team at Dechra, I now have first-hand industrial experience relating to pharmaceutical research and development, alongside a strong network of industry professionals that have already contributed greatly in my future career. It is with both the confidence and knowledge foundation gained through this internship that I can decisively progress my career toward a role in veterinary pharmaceutical product development."
Engagement and Committed Workforce
Informing and engaging our employees through internal channels of communication is of utmost importance to the Group. We have multiple channels of communication to provide both formal and informal updates including a Group newsletter that is issued twice a year (following the half-yearly and year end results), intranets, management and team meetings at the respective business units. These keep our employees informed of the financial performance of the Group, as well as the sharing of updates which are relevant to all Group employees such as management and team changes, progress in relation to strategic objectives and updates on corporate social responsibility objectives.
At Dechra, people are our greatest asset. In order to continue to retain our qualified and skilled employees, and to attract new employees we conducted an Employee Engagement Survey in March 2018 using the Great Place to Work (GPTW) survey. The results of the survey were disclosed in the 2018 Annual Report.
Given the diverse nature of our workforce, due to the geographical spread and the differing roles and segments in which our employees operate, we were very pleased with the overall results of our first survey.
Since the survey took place we have spent time communicating the results to our employees. Initially, we produced a short video with the overall highlights of the survey, and this was followed with feedback of the results at a business unit, department, site or country level utilising any key meetings with employees or team briefings.
Action planning took place with employee groups across the Group where employees had the opportunity to identify areas that they wanted to address as a result of the survey and we built a database of plans, predominantly led by the employee groups. A huge variety of approaches has been taken depending on the size of the teams and their types of issues.
We have scheduled a second Group-wide survey for March 2020 where we hope to see maintained or improved scores.
During the year, Lisa Bright has been appointed as the Non-Executive Director designated for employee engagement. She is currently investigating, along with the Group HR Director, the most effective way in which the Board can engage with our employees to readily understand their views. During the forthcoming year, an employee engagement forum will be piloted.

Engagement Survey
Announcing the engagement survey
A number of communications were shared with our employees globally to inform them that the engagement survey would be launched in March 2018. The communications asked our employees to provide open and honest feedback.
Aiding the understanding of the survey
Managers also held briefing sessions with their teams to share the GPTW presentation to build an understanding of why Dechra was launching the survey. We also used posters across our sites as a reminder to our teams of the importance of their input.
Employee participation
Following the completion of the engagement survey, videos were posted to thank our teams for their input.
Case Study
DVP EU
The EU team had a strong set of results with an overall response rate of 88% of their employees and a trust index of 76% (+9% higher than the Dechra Average score). The strengths of the EU survey were Culture (85%), Engagement (83%) Job Security (81%), Teamwork (80%) and Wellbeing (74%).
Following the feedback of the results,the DVP EU Senior Management team chose to focus on improving the area of Communication and Involvement (66%) across their region. Several work streams were created to focus on improving the level and frequency of communication both formal and informal. These include more focused use of the intranet for sharing news, creation of a monthly newsletter, regular sales updates to all staff, updates on the strategy from Tony Griffin and communication of the new EU marketing plan. More frequent conference calls have been put in place to allow Tony to talk directly to the EU teams, and the team has been utilising external social media such as Linkedin to promote our employee brand.
In addition to this, each of the Country and Functional managers within DVP EU has also undertaken the same action planning process with the support of the HR team and had their own action plan documented. Activities vary from additional training sessions, creation of dashboards to keep teams informed, bi-weekly town hall meetings and arranging cross-functional meetings on a quarterly basis to promote better alignment.
Culture of Safe Working Practices
Tony Griffin is the nominated Director responsible for health, safety and environmental matters. The Group attaches great importance to the health and safety of its employees and the public. The safety of our employees is paramount and that means continuing to reinforce good safety management practices as well as raising awareness of improved ways of working. Management are responsible for, and committed to, the maintenance, monitoring and promotion of a policy of health and safety at work to nurture the care and wellbeing of our employees, contractors and on-site visitors.
We have seven manufacturing facilities worldwide, employing 748 people representing 42.7% of our total workforce. Due to the nature of their roles, we have identified these as our higher risk employees with regards to health and safety. We have recently appointed a Group Dechra Pharmaceuticals Manufacturing (DPM) Health and Safety Manager who will initially be overseeing four of the main manufacturing facilities with the remit of standardising our procedures and working to ensure that high standards of health and safety are maintained.
To continue to improve the safety performance across both existing and newly acquired facilities, and to reflect the priority that is given across the business to safety, a proactive hazard awareness reporting initiative is in place in DPM.
Risk assessments are undertaken at our DPM sites to identify hazards and apply control measures to reduce or eliminate the risk of injury. Risk assessments may be conducted internally for routine activities or external specialists may be requested to conduct risk assessments for safety critical tasks. Where hazards are identified, these are scored according to the likelihood of an injury or incident occurring and the potential severity of this. Control measures are applied to reduce the risk of injury according to a hierarchy of control. We would firstly assess if a task was necessary and look for other safer ways to do a task before progressively applying other control measures.
All employees, contractors and visitors across the DPM sites are requested to remain vigilant at all times and to report any non-routine hazards they see using the local reporting procedures. We encourage employees to act immediately to warn others and control immediate risks whilst a more permanent solution may then be required to prevent the hazard from recurring. Because we work in a dynamic environment, we believe an increase in hazard reporting is an important leading indicator of the maturity of our safety culture.
DPM encourages local hazard reporting by both employees and contractors. The purpose of each report is to capture information about hazards and to track each hazard to an effective closure. It also allows us to provide feedback to the employees who have raised the hazards that action has been taken.
Each site periodically reviews any hazards raised and looks for trends. In addition to tackling each individual hazard, trend analysis allows each site to focus safety interventions on particular topics. This may include targeted safety training or safety communications.
For a number of years the Group has reported Lost Time Accident Frequency Rate (LTAFR) as a non-financial key performance indicator. A LTA is any absence or the inability of workers to conduct their full range of their normal working activities for a period of more than three working days after the day when the incident occurred. Any acquisitions during the year are included from the first full month that they become part of the Dechra Group. Despite maintaining a rigorous focus on health and safety, over the course of the last 12 months the number of incidents has increased from nil to six. All six incidents occurred in our manufacturing facilities; there were no fatalities. Two of the manufacturing facilities, Bladel and Melbourne, have now had over 24 months without a LTA.
All accidents and incidents are investigated by Line Managers with the cooperation of safety representatives or other employees who are aligned to an area. When an accident occurs, each site conducts an investigation which aims to identify the root cause of the incident including any workplace hazards, system or behavioural errors. Corrective and preventative actions are then implemented.
Any material health and safety issues or incidents that occur are discussed in detail at both business unit senior management meetings, and PLC Board meetings. Discussions include details of incidents and any remedial action taken to mitigate or prevent recurrence. Twice a year a comprehensive health and safety report is presented to the PLC Board meeting for discussion and review by the Directors.
We are routinely investing in safety, and during the 2019 financial year this has included:
- Refurbishment of floors in our Melbourne facility to reduce the risks of slips and falls;
- The purchase of new equipment to reduce the risk from manually handling heavy drain covers in Zagreb and the installation of a mist shower for personal decontamination;
- The installation of three defibrillators at our Skipton facility, along with the provision of first aid training to 40 employees; and
- An independent health and safety report was conducted at our site in Sydney, Australia with findings being implemented.
Group Causality
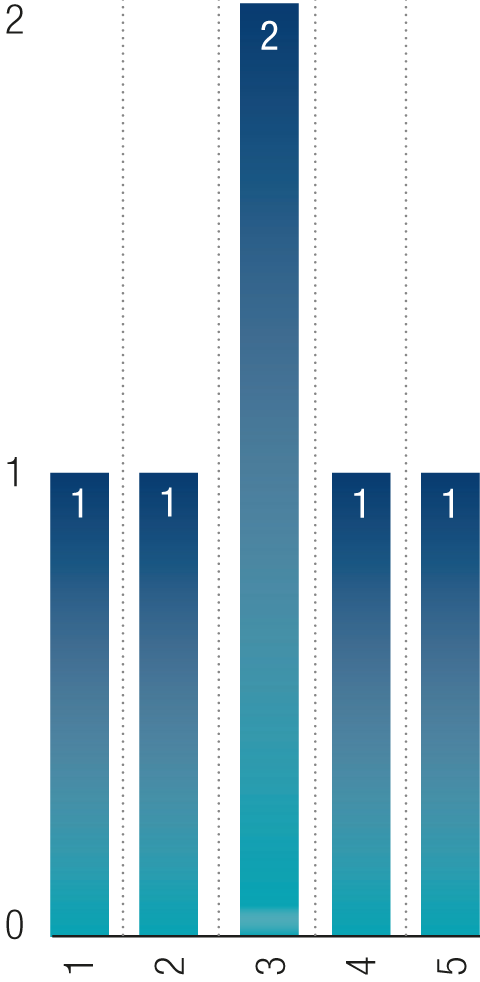
- Slipped/tripped or fell on the same level
- Ergonomic injury
- Contact with moving machinery or material being machined
- Contact with a hot/cold surface or substance
- Hit something fixed or stationary
DPM Hazards (Bladel, Florida, Skipton, Zagreb)
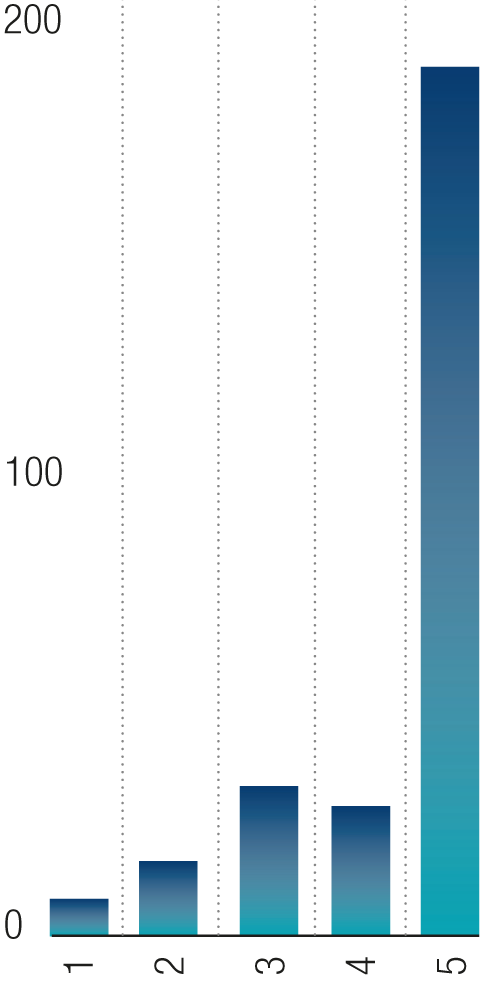
- Reportable >7 days
- LTA
- Investigated accidents
- Investigated (damage only)
- Minor or First Aid accidents
Case Study
Health and Safety Training
Working to ensure that our employees understand their health and safety obligations is critical to maturing and stabilising our safety culture. Employees receive general health and safety training at various stages:
- Induction – all new employees receive a health and safety induction on day one of their employment. This means that they understand the site emergency procedures, key health and safety rules and any welfare arrangements. This is supplemented in their departments with a local area induction.
- Job Training – Employees receive regular health and safety training according to their role and any hazards that exist in the work they are conducting. Role specific training, for example safe use of equipment, is delivered as part of operator training within their departments.
- General Health and Safety Training – General Health and Safety training is sometimes delivered to whole employee populations. Health and Safety Theme of the Month was introduced at Skipton in 2019 and is an opportunity to build awareness and competence around safety topics that are likely to be relevant to everyone working at a site. Topics such as Fire Safety, Manual Handling, Personal Protective Equipment and Workplace Transport and Pedestrian Safety are just some of the topics which have been covered.
- Subject Matter Experts – Across the Group there are many employees who are trained in specialist health and safety roles. These roles support the overall health and safety management system to enable risks to be controlled on a day-to-day basis. Examples of such roles are first aiders, fire wardens, and spillage responders. For each of these roles training is provided and this is refreshed according to the required frequency.
- Post-accident reviews – following any accident the need for retraining is reviewed as part of the accident or incident investigation.
